Artificial Disc Replacement
An artificial disc replacement is a procedure in which the soft cushioning between the bones of the spine, or vertebra, are replaced with a disc prosthesis or arthoplasty device in order to help carry loads and allow normal motion, much like the original disc. The two most common types of artificial disc replacement are total disc replacement (in which all of the disc tissue is removed and replaced with an implant in the same place) and disc nucleus replacement (where only the center of the disc is removed and replaced). Nucleus replacement devices are still under experimental investigation.
An Alternative to Spinal Fusion
For spinal pain, spinal fusion was traditionally considered to be the treatment of choice. Spinal fusion involves the removal of disc tissue and replacement with bone. The new bone is fused to the vertebra around the painful disc. This procedure eliminates movement in that area with the goal of minimizing pain. Spinal fusion is considered the gold standard in the treatment of painful spine conditions. However, there are concerns of adjacent level degeneration of the spine next to the fusion due to transfer of forces next to a fusion. Thus, using artificial disc replacement is a preferable alternative to spinal fusion, as the smooth, curved surfaces of the prosthesis, as well as the flexible nature of the devices, allow for more natural movement and hopefully less risk of adjacent level degeneration. Clinical outcomes comparing fusion and artificial disc replacement are similar, but the literature does support artificial disc replacement leading to less repeat surgeries regarding adjacent level degeneration.
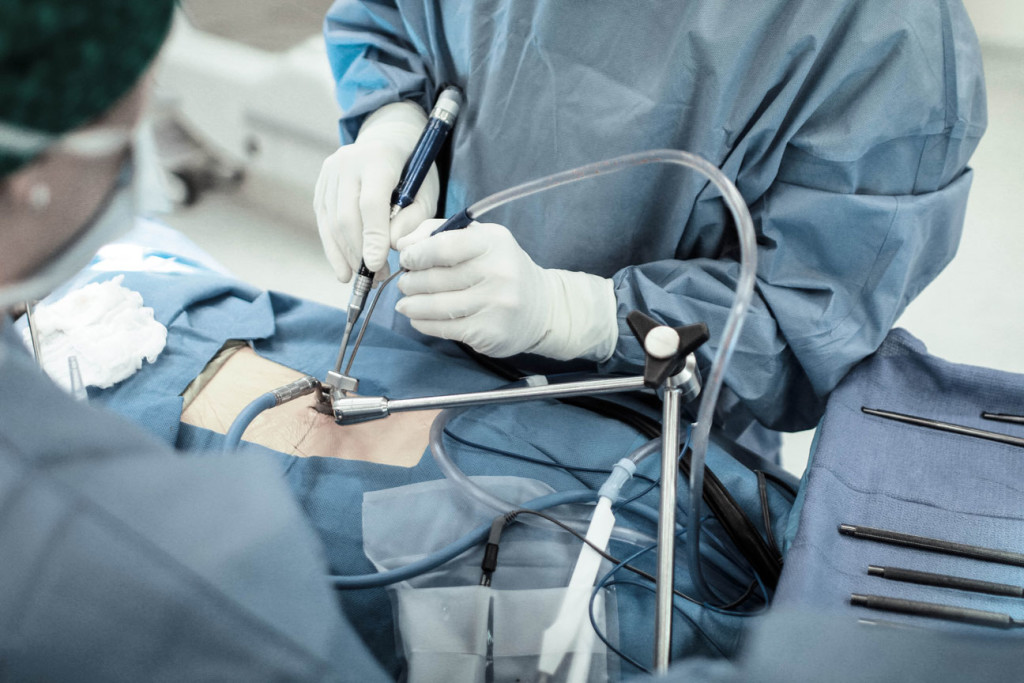
The Procedure
Artificial disc replacement surgeries in the neck or low back are performed the exact same way as traditional fusion procedures. The main purpose of both procedures is to remove the disc that is painful and/or putting pressure on the surrounding neural structures. Once the disc is removed, the fusion procedure involves placing spacers that promote fusion. The artificial disc replacement is a spacer that promotes motion, not a fusion. The incision is typically in the front of your neck or abdomen. The neck incision is small, approximately 1 inch. The abdominal incision is typically 4-5 inches. Surgery takes approximately 1-2 hours. Neck surgery patients usually go home within 24 hours. Low back patients will go home 1-2 days after surgery.
